ANDHRA PRADESH MEDTECH ZONE LTD
Ultra Modern Medical Equipment Manufacturing and Research hub welcoming Manufacturers & Innovators.
ANDHRA PRADESH MEDTECH ZONE LTD
Ultra Modern Medical Equipment Manufacturing and Research hub welcoming Manufacturers & Innovators.
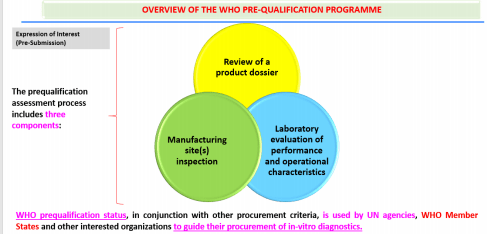
WHO announced AMTZ as a Support Cell for WHO Prequalification for In-Vitro Diagnostics (IVDx) on November 22nd 2017.The Cell would guide the IVD manufacturers from India and SEAR countries in getting their products prequalified and share the WHO expectations in this regard with manufacturers. This list of prequalified IVDx is used by international procurement agencies like UN Countries and WHO Member States and increasingly by countries to guide bulk purchasing of products.

Assistance in filing of application for WHO-PQ for all in-house indigenous manufacturers producing priority disease in-vitro diagnostics as per the listing of WHO. Complete hand holding for all in-house indigenous manufacturers